Does your child live with a rare disease called primary hyperoxaluria (PH)?
You may be interested in the PHYOX™ clinical trial program, a series of research studies evaluating a new investigational medicine called nedosiran, formerly DCR-PHXC, for the treatment of patients with PH.
For more information on the trial, visit clinicaltrials.gov and enter the identifier number NCT05001269.
Residents of the EU can visit EudraCT and enter the identifier number 2021-001083-16.

Is My Child Eligible?
To participate in the PHYOX8 clinical trial, the following criteria must be met:
- Newborn to 11 years old
- Must not be currently receiving dialysis, or requiring dialysis during the study period
- Documented diagnosis of either PH1 or PH2
- No history of or current need for organ transplant

If your child has PH1 or PH2 and you would like to learn more about the PHYOX™ clinical trials, fill out the form below.
A study representative will contact you and answer any questions you may have. Your information will only be used for the purpose of this study unless authorized otherwise.
Additional requirements will apply and will be discussed with you by study staff. Eligibility will be determined by a study doctor.
What to Expect
Approximately 25 participants will be enrolled in the study to receive monthly doses of nedosiran for a 6-month period. The study goal is to enroll a minimum of each in the following age groups.
The figure below depicts some of the activities a participant can expect at each study visit:
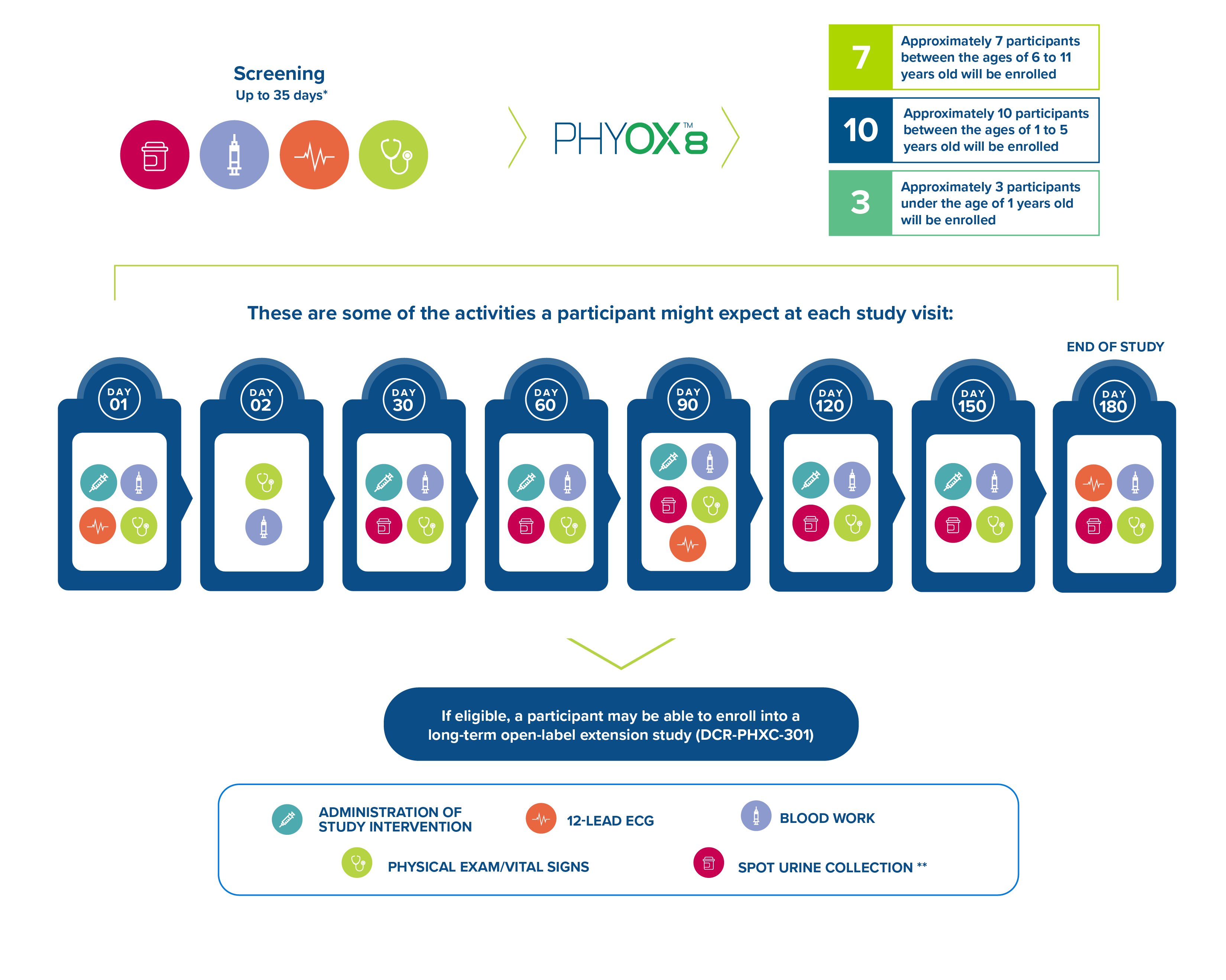
*An additional 14 days of screening may be required for further testing.
**Spot urine collection will include 6 samples at Screening and 4 samples at various times throughout the study visits.
In addition, a participant can expect to have a kidney ultrasound and echocardiogram at Screening and Day 180 of the trial. After completion of the PHYOX8 study, participants may be eligible for the PHYOX3 study for long-term follow-up.
About Primary Hyperoxaluria
Primary hyperoxaluria (PH) is a family of severe, ultra-rare, genetic liver disorders characterized by the overproduction of oxalate, a natural chemical in the body that is normally eliminated as waste through the kidneys. For more information on PH, click here.
About Dicerna
Dicerna Pharmaceuticals, Inc., a Novo Nordisk company, is focused on discovering, developing and commercializing medicines that are designed to leverage ribonucleic acid interference (RNAi) to silence selectively genes that cause or contribute to disease. Between Dicerna and our collaborative partners, we currently have more than 20 active discovery, preclinical or clinical programs focused on cardiometabolic, viral, chronic liver and complement-mediated diseases, as well as neurodegenerative diseases and pain.
For more information on Dicerna’s Privacy Policy, click here.
Safety Information
Nedosiran, formerly DCR-PHXC, is an investigational drug intended for the treatment of patients with PH. Investigational drugs are medicines that have not yet been approved by appropriate regulatory agencies. The safety and efficacy for nedosiran has not been established yet. Current and future studies are evaluating the safety and efficacy for nedosiran.
©2021 Dicerna Pharmaceuticals, Inc. / Lexington, MA



 English
English Deutsch
Deutsch Italiano
Italiano Polski
Polski Español
Español