Is Your Child Living With PH?
Does your child live with a rare disease called primary hyperoxaluria (PH)?

You may be interested in the PHYOX™ clinical trial program, a series of research studies evaluating a new investigational medicine called nedosiran, formerly DCR-PHXC, for the treatment of patients with PH.
For more information on the trial, visit clinicaltrials.gov and enter the identifier number NCT05001269.
Residents of the EU can visit EudraCT and enter the identifier number 2021-001083-16.
Is My Child Eligible?
To participate in the PHYOX8 clinical trial, the following criteria must be met:

If your child has PH1 or PH2 and you would like to learn more about the PHYOX™ clinical trials, fill out the form below.
A study representative will contact you and answer any questions you may have. Your information will only be used for the purpose of this study unless authorized otherwise.
"*" indicates required fields
What to Expect
Approximately 25 participants will be enrolled in the study to receive monthly doses of nedosiran for a 6-month period. The study goal is to enroll a minimum of each in the following age groups.
The figure below depicts some of the activities a participant can expect at each study visit:
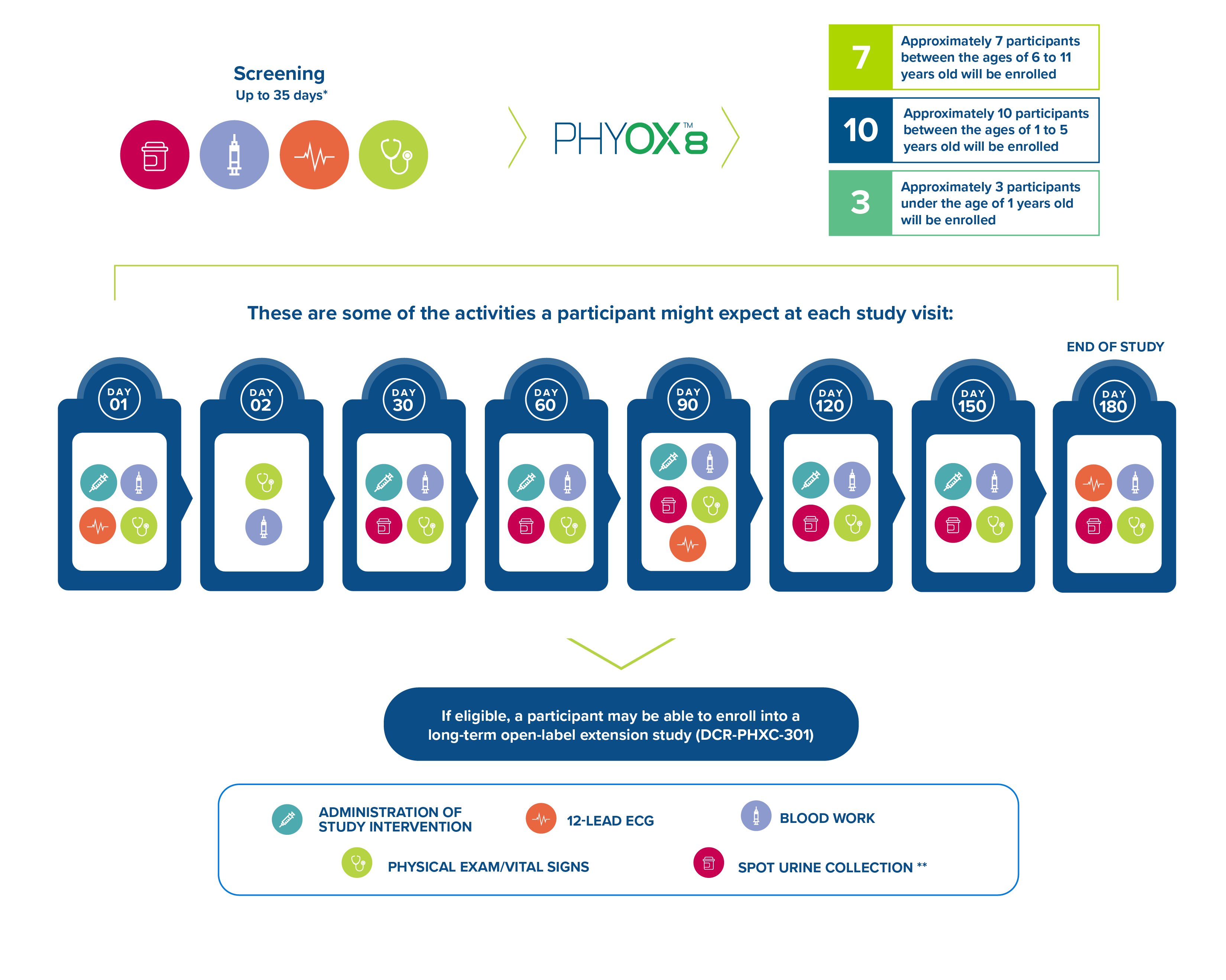
*An additional 14 days of screening may be required for further testing.
**Spot urine collection will include 6 samples at Screening and 4 samples at various times throughout the study visits.


